pH Nanosensors to Assess Viability of Transplanted Cells
In cases where patients under go therapeutic cell transplantation treatments, it is essential to monitor and ensure the viability of these transplanted therapeutic cells inside the patient’s body. Researchers at Johns Hopkins University in Maryland have created non toxic pH nanosensors to assess the cell viability in transplanted cells.
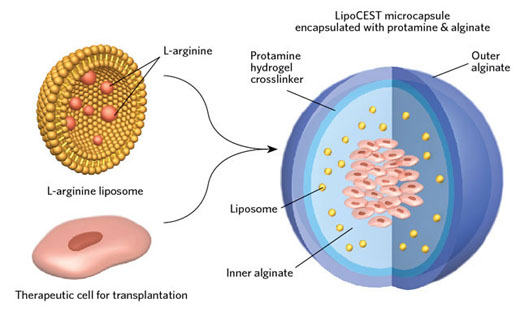
Before transplanting, the cells are encapsulated inside hydrogel microcapsules in order to localize them near the injection site inside the human body, and to prevent the host’s immune system from attacking these cells. Once these cells are implanted, it is almost impossible to safely assess the long term survival and functionality of these cells. The newly developed cell viability pH nanosensors can be easily incorporated into the hydrogel to address this issue.
The new pH nanosensors are made of lipid nanoparticles containing L-arginine, an excellent contrast agent that enhances visualization for CEST-MRI (Chemical Exchange Saturation Transfer). The degree of contrast exhibited by the compound is directly dependent on the pH of its surroundings.
The viability of cells affects the pH of the surroundings. As the cell viability decreases, the surroundings turn acidic and decreases the pH. This phenomenon is exploited by the L-arginine based cell viability nanosensors. CEST-MRI is used to check the viability of transplanted cells encapsulated with nanosensors. As the cell viability decreases, the local pH also decreases resulting in reduced contrast signal from L-arginine based nanosensors which is detected by CEST-MRI.
Tests were conducted on these pH nanosensors by encapsulating them along with liver cells in hydrogel microcapsules which are then transplanted into mice. As the cell viability decreased, a noticeable drop in pH was observed. When the cell viability reduced to zero, the CEST signal strength fell by 33 percent. The details of this research is available in the journal Nature Materials.