Types of Covid Vaccines and How Soon Can You Get Inoculated
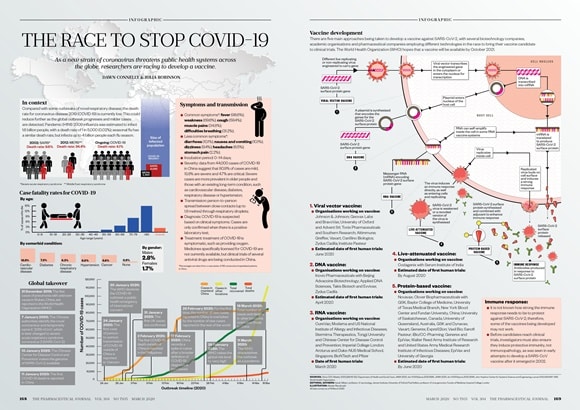
Pharmaceutical companies around the world are rushing to manufacture and distribute a viable vaccine. There are five main approaches being taken to develop a vaccine against SARS-CoV-2/COVID19, with several biotechnology companies, academic organisations and pharmaceutical companies employing different technologies in the race to bring their vaccine candidate to clinical trials. The World Health Organization hopes that a vaccine will be available earliest by October 2021.
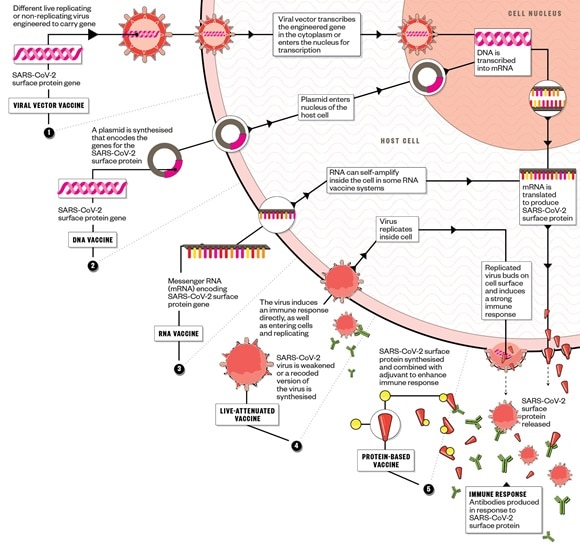
1. Viral vector vaccine:
- Organisations working on vaccine:
Johnson & Johnson; Geovax Labs and BravoVax; University of Oxford and Advent Srl; Tonix Pharmaceuticals and Southern Research; Altimmune; Greffex; Vaxart; CanSino Biologics; Zydus Cadila; Institute Pasteur - Estimated date of first human trials: June 2020
2. DNA vaccine:
- Organisations working on vaccine:
Inovio Pharmaceuticals with Beijing Advaccine Biotechnology; Applied DNA Sciences, Takis Biotech and Evvivax; Zydus Cadila - Estimated date of first human trials: April 2020
3. RNA vaccine:
- Organisations working on vaccine:
CureVac; Moderna and US National Institute of Allergy and Infectious Diseases; Stermirna Therapeutics, Tongji University and Chinese Center for Disease Control and Prevention; Imperial College London - Date of first human trials: March 2020
4. Live-attenuated vaccine:
- Organisations working on vaccine:
Codagenix with Serum Institute of India - Estimated date of first human trials: By August 2020
5. Protein-based vaccine:
- Organisations working on vaccine:
Novavax; Clover Biopharmaceuticals with GSK; Baylor College of Medicine, University of Texas Medical Branch, New York Blood Center and Fundan University, China; University of Saskatchewan, Canada; University of Queensland, Australia, and Dynavax; Vaxart; Generex; ExpreS2ion; Vaxil Bio; Sanofi Pasteur; iBio/CC-Pharming - Estimated date of first human trials: By June 2020
Immune response:
- It is not known how strong the immune response needs to be to protect against SARS-CoV-2; therefore, some of the vaccines being developed may not work;
- Before candidates reach clinical trials, investigators must also ensure they induce protective immunity, not immunopathology, as was seen in early attempts to develop a SARS-CoV vaccine after it emerged in 2002.
References:
Sources: China CDC Weekly 2020;2(8):113-122; Department of Health and Social Care; JAMA 2020, doi: 10.1001/jama.2020.1585; JAMA 2020, doi:10.1001/jama.2020.2648; John Hopkins Center for Systems Science and Engineering; Lancet 2012;12(9):687–695; World Health Organization

