Nanopore Technology Could Desalinate Seawater
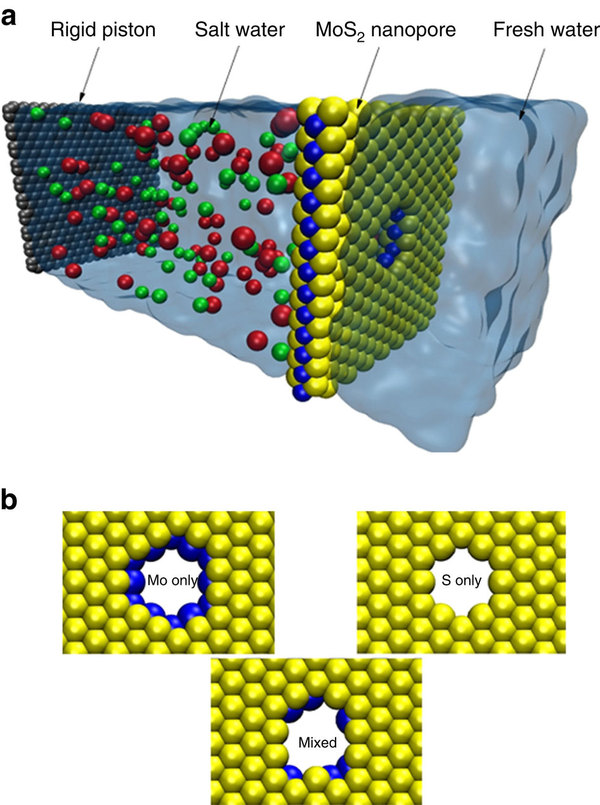
Simulation box (a) and pore architectures (b), among which Mo only pores are the most efficient. Credit: Nature Communications.
An ultra-thin sheet of molybdenum disulfide (MoS2) could let water molecules pass through tiny nanopores at a high rate while keeping ions and any contaminants. The authors of the study, a research team from the University of Illinois, ran desalination simulations for several materials in a supercomputer and concluded that MoS2 was the most efficient, 70% better than graphene. The finding was published in the journal Nature Communications.
Drinkable water makes a very small portion of the total water on earth. Water scarcity affects every continent: 1.2 billion people don´t have access to clean drinking water, and 2.8 billion have water shortages at least one month out of every year. Low-cost methods for purifying seawater are being sought after by many research group, as reverse osmosis (RO) is expensive. In RO, seawater is pushed through nanoholes in a plastic membrane that let only water molecules in. Due to the high pressure needed to push the water molecules and the constant pore clogging, an efficient alternative to RO is needed. Recent advances in nanotechnology open the door to designing filtration membranes with new materials: nanopores with diameters smaller than a salt ion can be practiced in ultra-thin membranes. Water flux is inversely proportional to the membrane´s thickness, so graphene layers as thick as an atom are much more efficient than conventional zeolite membranes. However, graphene layers must be functionalized with hydroxyl groups near the pores to attract water molecules and increase water flux. Functionalization is expensive and it reduces desalination efficiency.
MoS2 nanopores for water desalination
The team led by Dr. Aluru had previously worked with MoS2 membranes for nanopore DNA sequencing, which performed better than nanopores in graphene layers. MoS2 membranes, 1 nm thick, have a central sheet of molybdenum sandwiched between two sulfur layers. A nanopore in a MoS2 membrane exposes a molybdenum ring that creates a water-attracting shape. Moreover, sulfur on the outside of the membrane pushes water away, increasing the flow rate. These natural characteristics make MoS2 membranes more cost-effective than graphene, as there is no need to functionalize them.
Dr. Aluru´s team decided to run a simulation to test the desalination performance of MoS2 membranes. They used the Blue Waters supercomputer at the National Center for Supercomputing Applications at the U. of I. Molecular dynamics simulations were done for different conditions of pore size, chemistry, geometry and applied hydrostatic pressure. The simulations confirmed that the MoS2 membrane rejects more than 88% of ions and transports water at a flux two to five orders of magnitude higher than that of any other membrane.
MoS2 membranes are a very promising device for the future of water purification and salt rejection. The U. of I. team is now establishing collaborations to test them experimentally.
Source: University of Illinois


