Bacteria can work as antibody factories
Scientists from Cornell University and New England Biolabs have engineered E. coli as an antibody expression platform. The molecular machinery necessary for antibody production was introduced in the bacteria, which produced full length IgGs in the cytoplasm. The findings have been published in the journal Nature Communications.
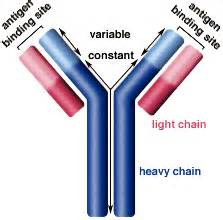
Monoclonal antibodies are proteins that recognize and destroy invasive elements in our bodies. They are widely used as therapeutic agents, and many research labs are constantly developing new ones. Currently, antibodies are mainly produced in hamster ovary cells, followed by mice cell lines and hybridomes. A small fraction of antibodies are produced in bacteria as antigen binding fragments (Fabs). Mammalian cell expression systems have advantages like greater expression and stability, but are expensive and slow compared to bacterial systems.
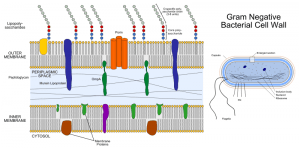
E. coli is a common platform for recombinant protein expression, but antibodies require the formation of a disulfide bond, which naturally occurs at the periplasmic space. Some research groups managed to produce the antibody chains and assemble them in the periplasm, but this method presents several disadvantages. forst, the periplasm is relatively small, so it can not support high antibody expression levels. Second, the periplasm lacks the chaperone proteins that assist antibodies to fold properly and thus be functional. Third, the antibody chains can not easily be delivered to the periplasm, as they need to cross the cytoplasmic membrane.
Creating a less reducing environment in the cytoplasm
Dr. DeLisa and Dr. Berkmen presented a new approach to these problems. Their teams engineered the E. coli strain SHuffle to make a more oxidative environment in its cytoplasm, similar to conditions found in the periplasm that allow disulfide bond formation. They successfully produced and assembled IgGs in the cytoplasm, where there are several chaperones to fold the antibodies. The method also avoids the energetic cost of membrane translocation. The production level of the so-called cyclonals was much higher than in the periplasm. The study also proves that the new technique can be combined with strategies commonly used to create custom antibodies with clinical properties, like grafting alternative epitope recognition domains, humanizing and Fc engineering.
The new platform will allow unprecedented antibody expression levels for therapeutic and diagnostic applications. The researchers have so far produced antibodies against avian flu virus, Bacillus anthracis and breast cancer.
Source: Cornell University