Cryo-Electron Microscopy Is the Method of the Year 2015
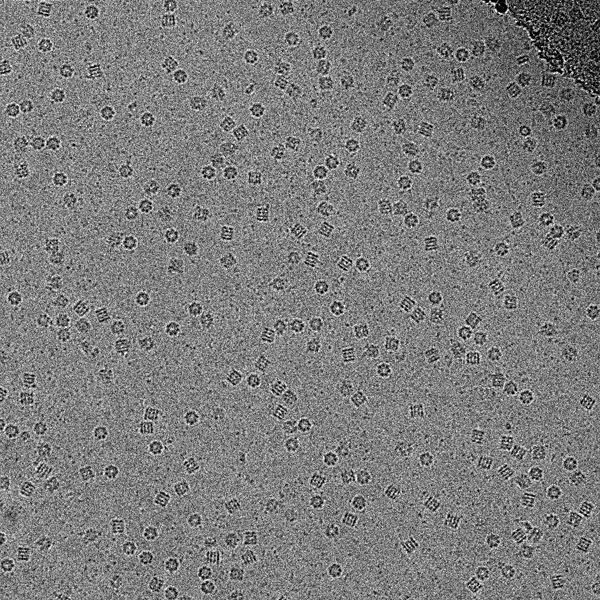
CryoEM image of GroEL, 50000x. Credit: Vossman, https://commons.wikimedia.org/wiki/File:Cryoem_groel.jpg
Nature Methods has named Cryo-Electron Microscopy the method of the year 2015. This structural technique, which was always a step behind nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography, is now capable of near-atomic resolution thanks to important technical advances.
Most protein structures have traditionally been solved by X-ray crystallography, but some proteins are not crystallizable. The common alternative for small proteins is NMR, but this technique can’t solve large proteins’ structures due to peak overlapping and broadening in the NMR spectrum. Cryo-EM is better suited to solve the structure of large proteins or protein complexes, but until recently it was a low-resolution technique. Fortunately, the technique has advanced over the last years, improving resolution and increasing its applicability to biological problems. New, highly sensitive detectors allowed to solve protein structures at 3-Å resolution this year. Sample preparation techniques have progressed too, as well as the software to process the images, but there is still a lot of room for improvement in these areas.
Cryo-EM is growing rapidly, and the demand for new instruments is not always reasonably met. Many countries are setting up structural biology units that include state-of-the-art cryo-electron microscopes, causing a shortage in their availability. An additional problem is the complexity of the technique, from sample preparation to data analysis, requiring a lengthy training with highly specialized personnel.
A thorough analysis of cryo-EM by Nature Methods
As the Method of the Year 2015, cryo-EM is the Special Feature of the January 2016 issue of Nature Methods. Several scientists contributed with articles on the history and future of cryo-EM, protein-labelling techniques for better imaging, desirable developments in 3D imaging to analyze chromatin structure and dynamics, advances in the observation of protein structural changes, new computational tools and single-cell and single-particle approaches.
Source: Nature Methods
