Damage-Free X-Ray Protein Crystal Studies
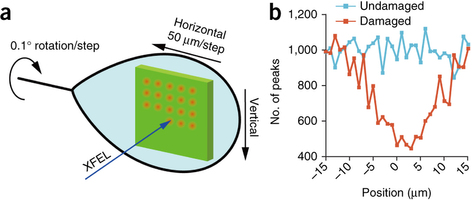
Experimental setup for femtosecond crystallography characterized by the combined usage of a large crystal and femtosecond XFEL pulses.
Researchers in Japan are reporting on a new damage-free technique of x-ray crystallographic studies, wherein instead of continuous bombardment with x-rays, the researchers record still diffraction patters at controlled rotation sequences with femtosecond pulses avoiding permanent radiation damage to the molecule.
X-ray studies require scattering of bean by the atoms of the crystals to identify their periodically repeating arrangement. The x-rays used for precise studies are of high intensities that end up causing permanent damage to the same proteins that are being studied.
Researchers at numerous universities across Japan and the SPring-8 angstrom compact free-electron facility used the powerful x-ray free electron laser (XFEL) to precisely study large complex protein molecules without causing any sort of radiation damage with the new technique. The molecule is targeted with a high intensity x-ray spot for brief femtoseconds with stepwise crystal rotation. By averaging (Monte Carlo approach) the patterns obtained from different rotation angles, the resulting structure of the protein obtained is of high quality.
A distance of 50µm was maintained between each successive x-ray exposure spot in the experimental studies on multiple crystals of bovine heart cytochrome c oxidase. The resulting structure obtained was precise to 1.9 A without causing any damage to the protein.
Reference: Determination of damage-free crystal structure of an X-ray–sensitive protein using an XFEL [link]
