FDA Allows Release of HIV Diagnostic Device
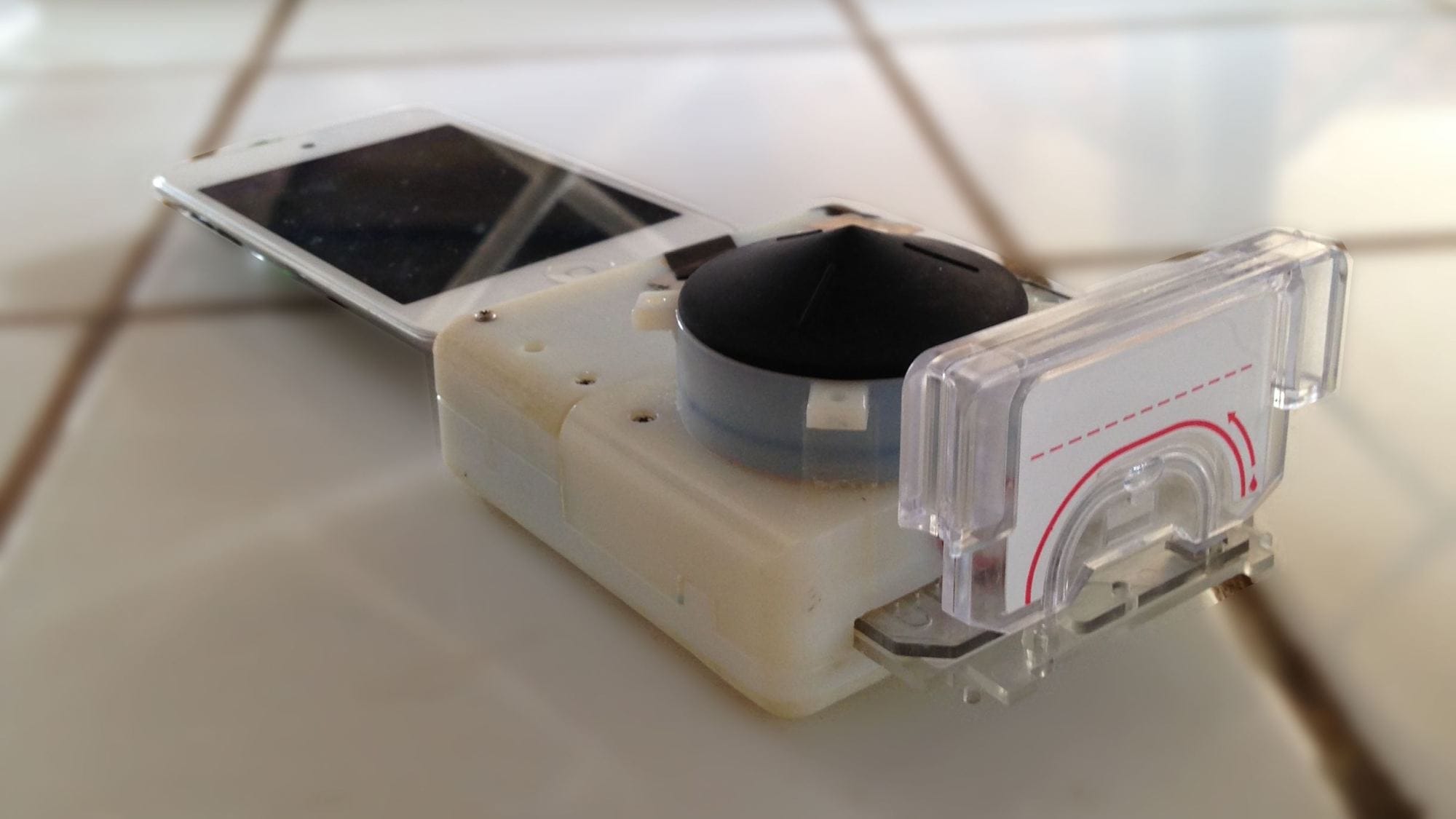
The medical technology company Beckton, Dickinson and Company (BD) has received 510(k) clearance from the Food and Drug Administration (FDA) to sell the FACSPresto™ and the FACSPresto™ CD4/Hb Cartridge, two systems designed to detect lymphocytes and hemoglobin in HIV/AIDS patients’ blood.
 The FACSPresto system is a 6 kg fluorescent imaging cytometer and absorbance spectrometer that obtains absolute and percentage counts of CD4 T lymphocytes and hemoglobin concentration from HIV/AIDS patients’ whole blood samples. The system uses single-use cartridges that contain dried reagents, increasing shelf life. The blood sample is deposited into the cartridge, which is then inserted in the reader. A user-friendly touchscreen interface allows language-independent interaction with the system, and results are obtained in four minutes.
The FACSPresto system is a 6 kg fluorescent imaging cytometer and absorbance spectrometer that obtains absolute and percentage counts of CD4 T lymphocytes and hemoglobin concentration from HIV/AIDS patients’ whole blood samples. The system uses single-use cartridges that contain dried reagents, increasing shelf life. The blood sample is deposited into the cartridge, which is then inserted in the reader. A user-friendly touchscreen interface allows language-independent interaction with the system, and results are obtained in four minutes.
Clinical studies have demonstrated that the accuracy, precision and linearity of the FACSPresto is comparable to that of classical analyzers. With its reasonable cost, the new device could be used in diverse clinical settings, helping to monitor HIV/AIDS patients and to control the spread of the disease.
Source: bd.com
