First Epigenetic Mechanism Involving Histone Tails Solved at Atomic Level
Researchers from the Institute for Research in Biomedicine (IRB Barcelona), the University of Cambridge, and New York University have discovered the structural mechanism of chromatin unfolding by epigenetic modifications. Acetylation of histone tails unfolds chromatin by decreasing tail availability and thus interactions between nucleosomes.The researchers ran computer simulations of nucleosome dynamics and experimentally confirmed the results. The findings have been published in JACS.
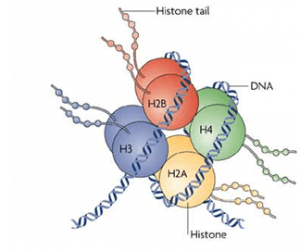
Histones are structural proteins that bind DNA. Every 147 DNA base pairs fold around an octamer of histones, creating a bead-like structure called nucleosome. Histones have their tails exposed, which suffer chemical modifications that affect their structure and that of the nucleosome. This changes in structure have very important effects in gene expression, as they affect DNA accessibility to the molecular machinery. The most common modifications are lysine acetylation and methylation. However, it is not known how these modifications cause the structural changes of chromatin. Technically, it is still complicated to obtain accurate, atomic level information about the mechanism of chromatin activation.
Molecular dynamics simulations and NMR measurements of chromatin
To know better the mechanism by which histone tails can activate or deactivate gene expression, Modesto Orozco from IRB and his colleagues ran molecular dynamics of pairs of nucleosomes and their tails in presence of solvents and ions. The computer models were later validated by NMR experiments. They also carried out simulations of 24-nucleosome arrays to describe the conformational landscape of histone tails, their roles in chromatin compaction, and the impact of lysine acetylation.
The researchers found out that histone tails become more structured upon acetylation. As a result, histone tails get shorter and can not engage in interactions with other nucleosomes. Thus, the general nucleosome landscape loosens up and DNA is more accessible to transcription factors and other proteins, that is, chromatin becomes more active.
It is the first time an epigenetic mechanism is elucidated at atomic detail. This work opens the possibility of explaining other epigenetic mechanisms and linking epigenetic modification, structural change and phenotypic effect.
Source: genengnews