IUPAC Verifies the Discovery of Four New Chemical Elements
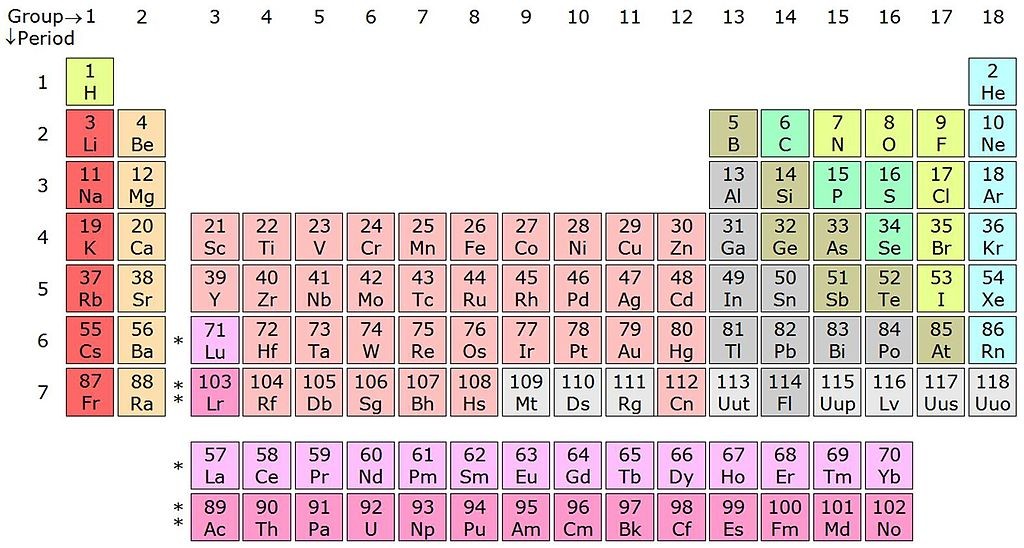
The International Union of Pure and Applied Chemistry (IUPAC) has reviewed the literature for elements 113, 115, 117 and 118 and has concluded that the claims for their discovery fulfill the criteria outlined by the IUPAC in 1991. The new elements complete the 7th row of the periodic table. The discoverers will be invited to suggest permanent names and symbols for these elements.
Chemical elements are classified according to their atomic number -the number of protons per atom. Elements with more than 92 protons are unstable and can be only obtained experimentally for an extremely short amount of time. The new elements would have been impossible to obtain without particle accelerators, which were used to propel light nuclei at heavy ones, transiently creating heavier elements.
The main difficulty in identifying these elements is their decay into unknown isotopes of lighter elements. Experts hope to have soon methods to directly measure the atomic number.
Temporary names and symbols
Element 113 (ununtrium, Uut) was discovered in the RIKEN lab in Japan. Elements 115 (ununpentium, Uup) and 117 (ununseptium, Uus) were discovered by the Joint Institute for Nuclear Research in Dubna, Russia, the Lawrence Livermore National Laboratory in California and the Oak Ridge National Laboratory in Tennessee. Element 118 (ununoctium, Uuo) was discovered by the Dubna and Lawrence Livermore research centers.
The new names have to be consistent and translatable into other languages. Elements can be named after a mythological concept, a mineral, a place or country, a property or a scientist.
Source: IUPAC