New Technique Reveals Dual Properties of Proteins Involved in DNA Repair
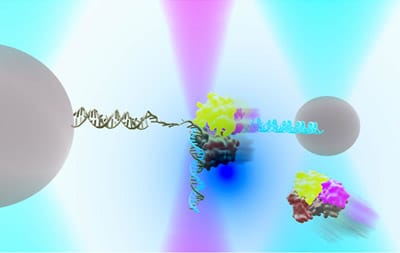
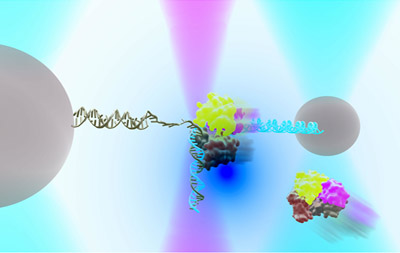
DNA repair in Ecoli with helicase UvrD can exist in an “open” or “closed” state. An instrument combining optical traps & a single-molecule fluorescence microscope is used to measure together the relationship between these two structural states & their functions in DNA repair.
University of Illinois researchers have discovered an avant-garde way of identifying the structure and functions of proteins using the combination of two innovative technique called simultaneous fluorescence microscopy and optical trapping.
Scientists until now have used two separate techniques to study structure of a protein and the role they play in the cell. It mostly follows the confirmation of the static structural form first, followed by its dynamic function next. Two techniques which are closely interrelated and whose roles can only be confirmed/deduced with the confirmation of the other.
Researchers at University of Illinois, biological physicists Taekjip Ha and Yann Chemla used this innovative technique to successfully identify the number of proteins of helicase UvrD involved in DNA repair. Helicase UvrD is an important protein in nucleus that plays an important role during repair. It unzips and unwinds DNA in Ecoli and is similar in function to helicase found in humans and involved in DNA repair as well.
Two Techniques Combined
While Ha is an expert at innovative single molecule fluorescence microscopy and spectroscopy techniques, which help identify protein structure, Chemla is the foremost expert in expert in optical trapping techniques. Researchers combined their respective expertise to create simultaneous fluorescence microscopy and optical trapping, which according to Ha, “yields far more definitive answers to questions relating structure to function than either technique could independently”.
Two Proteins involved in DNA Repair
The findings have been published in Science, “Direct observation of structure-function relationship in a nucleic acid–processing enzyme”. To identify the number of proteins involved in unwinding and unzipping, the researchers added two different dyes on each protein and watched using an optical trap. The researchers noticed that when only one protein was involved, the unwinding was only partial, however when two proteins were involved, the unwinding happens to a larger extent therefore helping .
Bioengineering Protein States
The helicase UvrD also has two positions, an ‘open’ and a ‘closed’. Researchers were also able to identify that molecules change forms between ‘open’ and ‘closed’ involved in DNA repair. The function of the ‘open’ and ‘closed’ forms was confirmed by creating a bioengineered proteins whose positions could be controlled.
The innovative technique was repeated on another similar protein called PcrA, published in science titled, “Engineering of a superhelicase through conformational control”. This technique helps identify the dual nature of proteins and how the structure and presence of certain molecules changes their function. ‘
Source: University of Illinois
