Synthetic Photosynthesis Produces Fuel Liquid from CO2
Scientists from the University of California at Berkeley have designed an artificial “plant” that can produce methane thanks to semiconducting nanowires and bacteria. In the synthetic photosynthesis, sunlight, CO2 and water are transformed into liquid fuel that can be stored and distributed through energy infrastructures. The findings were published in the Proceedings of the National Academy of Sciences (PNAS).
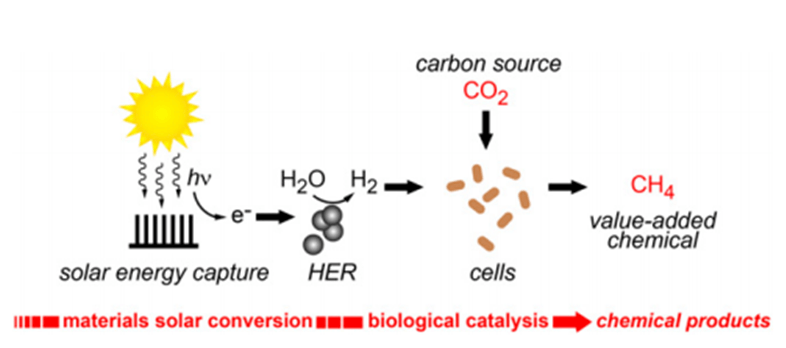
Synthetic photosynthesis is a technology based on natural photosynthesis that produces clean energy. Instead of producing sugars from CO2, water and light, the synthetic version seeks to obtain liquid fuels. This technology has a great potential: it could mean the end of the oil industry and the start of a new era of clean production of gasoline and natural gas. Synthetic photosynthesis is still a young technique, and there are many problems to solve before it becomes common. But Dr. Peidong Yang and collaborators have given a huge step: they created a hybrid inorganic/biological system to produce methane. Moreover, the system, based on bacterial enzymes and nanoconductors, helps researchers understand better the photosynthetic process and design better artificial processes based on it. The final aim is to construct a totally synthetic system, more robust and efficient than the natural one.
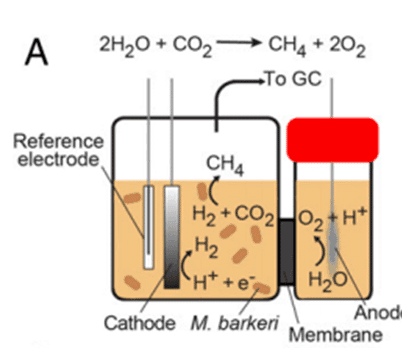
Dr. Yang´s team presents in their last paper a hybrid, bioinorganic solar-to-chemical conversion system. Electrons from a light source are used by the biocompatible, inorganic hydrogen evolution reaction (HER) catalyst to transform water into hydrogen molecules. The HER catalyst acquired photochemical properties when it was structured as a film coating a semiconductor photocathode. Thick films confer higher water-splitting activity, whereas thin ones capture more photons.
In the following step, the bacteria Methanosarcina barkeri reduces atmospheric CO2 using the inorganically generated H2 as an electron donor, to finally produce methane (CH4), the principal component of natural gas.
This hybrid system could be used as a model for future complexes, combining other biological and inorganic components to produce different chemical products.
Source: EurekAlert!