Electron Holography Microscope Obtains First Image of a Single Protein
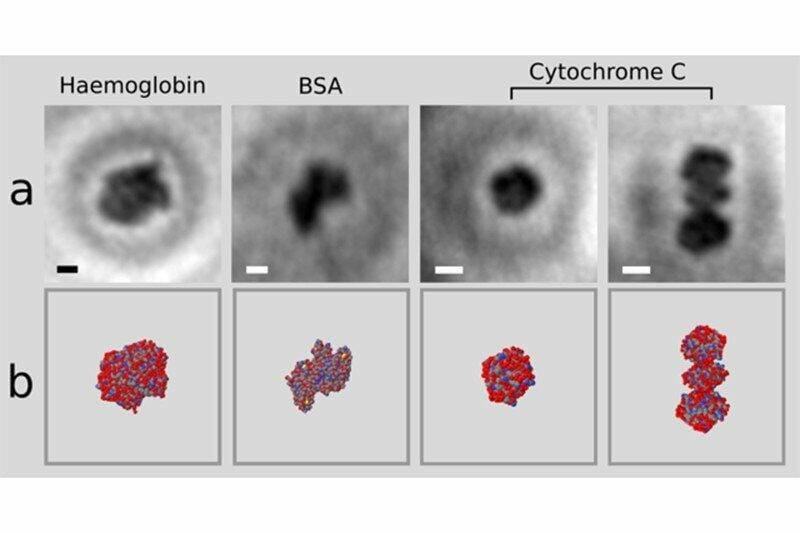

Electron holographic microscopy of single proteins (a) and X-ray structures from multi-protein samples (b) Credit: Jean-Nicolas Longchamp
Researchers from the University of Zurich have obtained the first image ever of a single protein. The molecule was fixed on a graphene sheet and imaged with an electron holographic microscope. By emitting low-energy electrons, this technique acquired images without damaging the sample, and the thinness of graphene allowed electrons to pass through it and reach the microscope detector. This is a major breakthrough in structural biology that will have a positive impact in understanding the relationship between protein structure and function, and will help understand the molecular mechanisms of diseases caused by mutations that alter DNA sequence and, subsequently, protein structure.
Protein function is determined by its structure. Current techniques obtain protein structures by averaging out the observations of a sample that contains multiple copies of the same protein. This is problematic, because proteins can adopt different conformations. As a result, the proteins in a crystallographic or cryo-electron microscopy sample are captured in different configurations, and the structure obtained will be an average of those multiple shapes.
X-ray crystallography has another limitation: not all proteins crystallize. In those cases, nuclear magnetic resonance (NMR) spectroscopy can be used. NMR structures are usually obtained from samples of proteins in solution, which is closer to their physiological state. The final model is actually an ensemble of structures, that only overlap when there is sufficient information to impose a particular fold. Unfortunately, NMR spectroscopy is usually unable to obtain structures of proteins bigger than 35 kDa.
These limitations have impulsed structural biologists to look for a way to image single proteins. The first requirement is a technique to isolate proteins individually. Second, the protein should be fixed for enough time to acquire the necessary structural information. And finally, irradiation should not damage the sample, but should be strong enough to capture all structural details. Jean-Nicolas Longchamp et al. have developed a technique that satisfies all these conditions.
Protein spray and soft landing on graphene; gentle irradiation of single proteins
The researchers used an electrospray beam to select and place individual proteins on a graphene sheet. Then, they irradiated the proteins with non-damaging, low-energy electron beams. The ultra-thin graphene allowed the electrons to pass trough the sheet towards the detector, located on the opposite side of the emission source. The information received by the detector is the interference pattern, a hologram of the protein. With this techniques, the researchers were able to obtain the first atomic-resolution holograms of single molecules of Cytochrome C and bovine serum albumin (BSA) and of the protein complex haemoglobin.
The techniques used in this study are relatively feasible for most structural biology laboratories. This could imply a widespread increase in electron holography use in single proteins in the near future.
Source: arxiv.org
