Protein 3D Structure Reveals New Information About Neurotransmitters Release
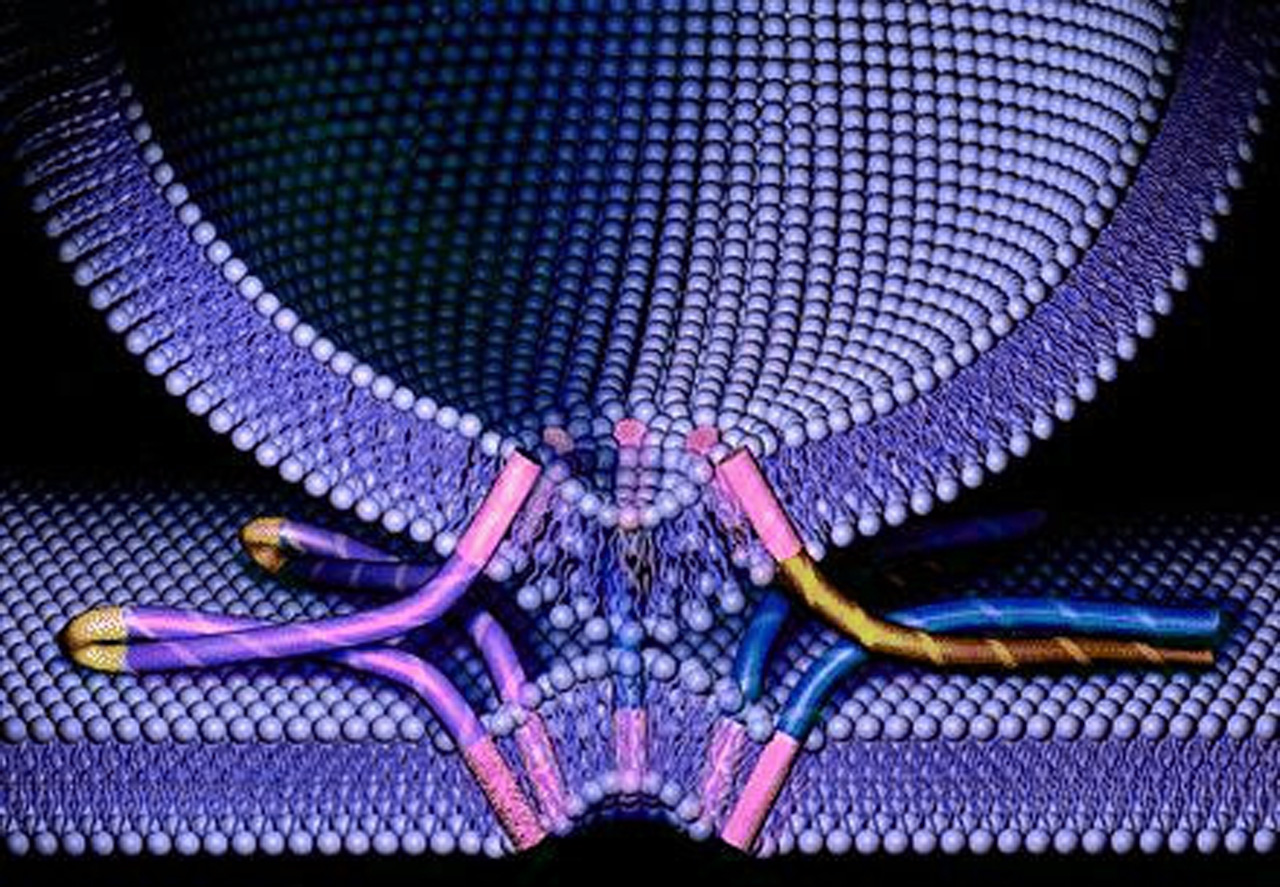
Scientists from the SLAC National Accelerator Laboratory, Stanford University and Stanford School of Medicine have solved the 3D structure of the SNARE-synaptotagmin-1 complex that controls release of neurotransmitters. The study has been published in the journal Nature.
SNAREs are a protein superfamily of more than 60 members in yeast and mammalian cells. Their role is to mediate vesicle fusion with their target compartment. In neurons they intervene in the docking of synaptic vesicles, loaded with neurotransmitters, with the presynaptic membrane, generating a response.
The researchers found out that, when the SNAREs and synaptotagmin-1 come together, they amplify the increase in calcium concentration, triggering a fast release of neurotransmitters. The two proteins already interact before joining the membrane, which increases the response speed.
The researchers had problems to crystallize the proteins, a common problem in many X-ray crystallography studies. With the help of a robotic platform at the Linac Coherent Light Source (LCLS) at the SLAC, they managed to crystallize the complex. Finally, they combined hundreds of X-ray images from 150 protein crystals. The atomic level resolution of the structure brought new information about the interaction sites between synaptotagmin-1 and SNARE.
This improved understanding of the neurotransmitters release mechanism could help in the design of drugs for disorders like depression, schizophrenia and anxiety.
